Editoriale
Metodologie didattiche per l'Università
-
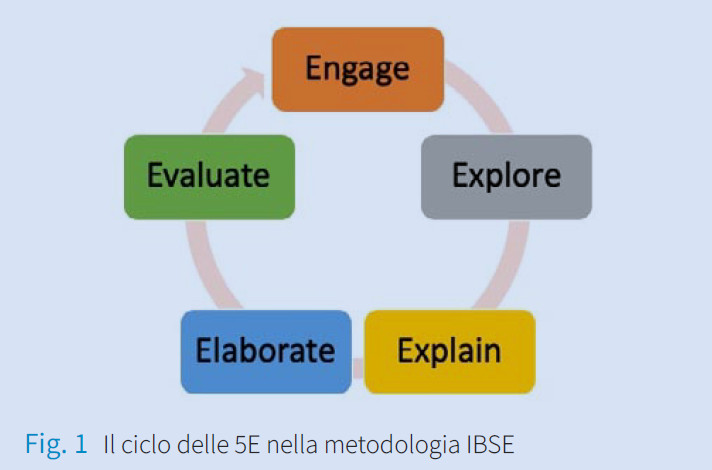
Si può costruire il concetto di periodicità senza il modello atomico di Bohr?
The concepts of atom, element, and substance, while constituting fundamental chemical concepts, are generally tricky to understand. The not authentic comprehension of these concepts results in their not appropriate use, in the association
of the idea of periodicity almost exclusively with the description of the atomic properties and the consequent limited use of the periodic table. Based on this evidence, we investigated the possibility of presenting periodicity’s concept without introducing atomic models and electronic configurations, while referring exclusively to the classification criteria of the elements identified by Mendeleev. As a result, a module of a vertical path aimed at developing the concept of periodicity was designed. Furthermore,
we planned and realized activities based on the macroscopic properties and composition of substances, which made it possible to obtain a first simple Periodic Table of elements. The project was proposed and carried out as part of a Master’s thesis in Didactics of Chemistry. This contribution will discuss the choice of the methodology, the strategy, and the tools employed in these activities. -
Il laboratorio nella didattica delle discipline tecnologiche (e scientifiche)
The article analyses the importance of the laboratory in making teaching effective. The reference is to electronics, but what is reported may also apply to other technological and scientific disciplines and, perhaps, to all school disciplines. The laboratory is important not only for the help it provides in the acquisition of new knowledge, but also because, from a more psychological point
of view, it creates in students a predisposition that encourages the desire to learn. -
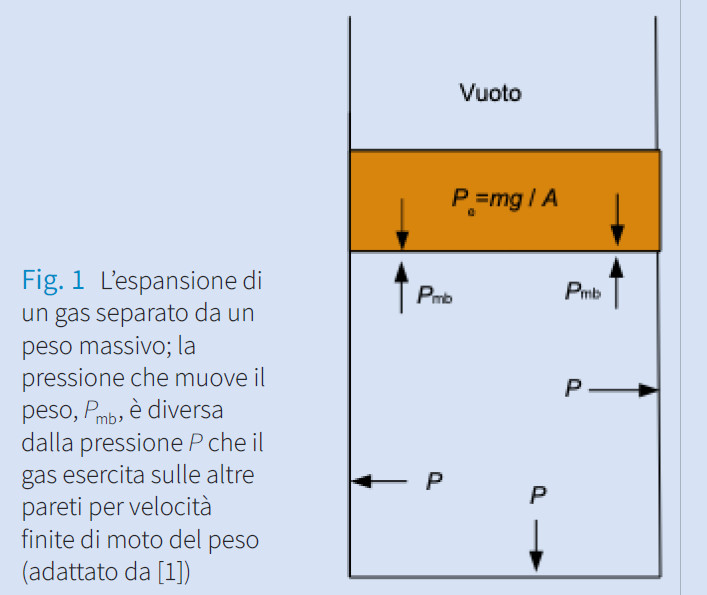
Il lavoro di espansione in termodinamica
In physical chemistry courses, the teaching of the expansion work is often conducted with reference to the external pressure. This practice, consistent with the equations of the dynamics of the material point only in special cases and for finite transformations, can be avoided without mathematical burden and by highlighting the difference between system-based work and surroundings-based work, which has an important conceptual and practical role.
Percorsi storico epistemologici per la scuola superiore
-
Decifrare la chimica invisibile: un excursus storico-epistemologico dai tubi di Crookes alla radioattività
The period between the last decades of the 19th century and the beginning of the 20th was characterized by extremely lively science, underpinning new subject areas in physics and chemistry. At the same time, the language of science went through a phase of great renewal. Central was the role of the discovery of radioactivity, the historical background of which is therefore extremely important for secondary school students. This phase of great progress generated collective euphoria, curiosity
about paranormal phenomena and a certain aura of mysticism, as proved by theatre and film shows, reports about mediums and strange scientific-theosophical mixtures.
Percorsi laboratoriali
-
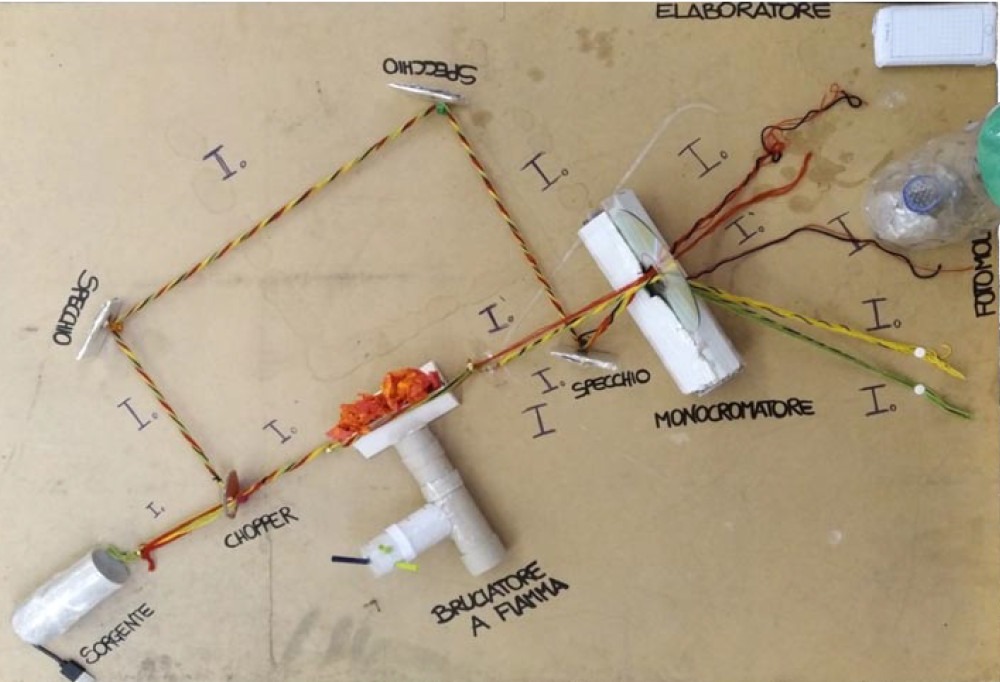
Il “percorso dei percorsi” sulla spettroscopia nella scuola secondaria di secondo grado: un’analisi dei lavori di gruppo presentati dai docenti partecipanti alla V Edizione della Scuola “G. Del Re”
This paper reports the analysis of several didactic sequences and educational activities designed and put into practice by high school teachers who attended the V Scuola Nazionale di Didattica della Chimica “Giuseppe Del Re” (2020 edition).
The teacher training course was focused on how to introduce spectroscopy at high-school level and it was organized in three steps: introduction of materials and resources by the “Giuseppe Del Re” trainers, design of educational activities specific
for different school types and students’ target, development and carrying out of the activities in class. The number and quality of educational activities, projects and obtained materials was very satisfactory and it resulted in several interesting approaches able to engage both teachers and students. In this paper, all these activities are commented and presented in a whole educational pathway about spectroscopy.
Musei scientifici e didattica
Pagine di storia
-
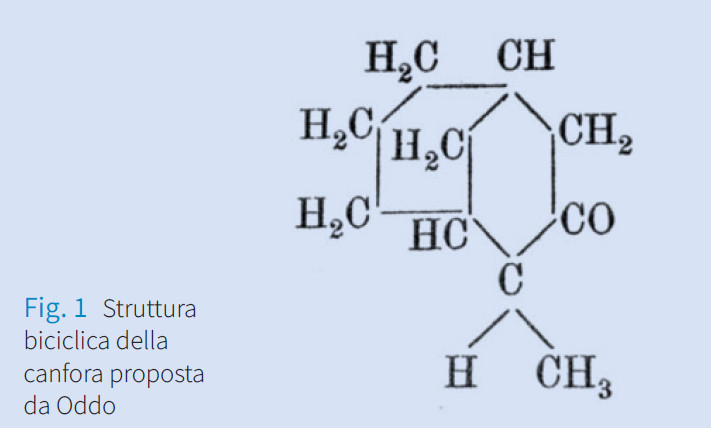
Giuseppe Oddo: chimico “rivoluzionario” e “irriverente”
Giuseppe Oddo can be considered a “revolutionary” and “irreverent” chemist. He was considered “revolutionary” by the reason of he proposed radically innovative positions in relation to traditional ones as well as “irreverent” because of his cultural independence and irritable character also led him to collide with many influential colleagues over the years. The feature that distinguished Oddo’s entire activity was his cultural independence, his ability to break out of pre-packaged schemes
that were not his own and to put forward innovative proposals and suggestive hypotheses. Oddo’s scientific output includes, among other things, the first bicyclic structure of camphor, the possibility that the hydrogen atom shared its valence with two
other atoms (mesohydria) and, the hypothesis that noble gases such as Krypton and Xenon were not inert.
Dare voce agli studenti
-
La scuola di oggi e il mondo di domani : insegnamento delle scienze, storia e studi della scienza (STS)
Articolo tratto da tesi di laurea di Giovanni Polisena, La scuola di oggi e il mondo di domani. Insegnamento
delle scienze, storia e STS, Bologna, Università di Bologna, 2022Scientific specialism is inevitable, but not in the field of teaching. Science, technology and society (STS) studies’ approach along with the history of science can equip students with the skills to face the complexities of society. The introduction of this teaching approach within upper secondary schools could represent the unifying element missing from the increasingly jagged scientific curricula. At school, it is important to educate students to debate scientific values, because these will determine the
political choices and social visions of tomorrow.
Keywords
-
Il legame chimico: dalla complessità quantistica alla modellizzazione didattica
Il legame chimico è uno dei concetti fondanti della Chimica, ciò nonostante esso resta un concetto polivalente, complicato, se non complesso. In esso, infatti, confluiscono varie componenti di difficile, se non impossibile, sintesi. Da un lato, rappresenta
il punto d’arrivo dei concetti del XIX secolo che i chimici avevano elaborato per spiegare che cosa tenesse insieme gli atomi, con successiva rivisitazione nell’ottica quantistica; dall’altro, tale concetto si deve rapportare con l’approccio fisico al mondo
microscopico e con le problematiche “non classiche” della sua descrizione. In mezzo, abbiamo la didattica della Chimica, la necessità di “tradurre” questa complessità per studenti di differenti età e cognizioni scientifiche.
In ricordo di Floriano
Esperienze di chimica e di vita vissuta
-
La mia scoperta dell’America: la mia scoperta dell’America La prima esperienza americana di un giovane ricercatore
The author tells his first American life experience as a researcher in an excellent Californian Research Laboratory. He has also the opportunity to report his emotions and those perceived in his meetings in New York on the occasion of the assassination of the President J. F. Kennedy which just coincided with his arrival in USA.