Editoriale
Metodologie didattiche per l'Università
-
Prospettive per il miglioramento della didattica universitaria dopo l’esperienza della pandemia: con.Scienze tenta un bilancio dell’esperienza della DAD
On September 24th, 2021, con.Scienze organized a one-day conference to reflect on the experience of on-line teaching. The conference featured trans-disciplinary contributions and disciplinary sessions, which allowed the sharing of good practices
and the discussion of the many critical aspects of online training. This is a short report of the conference and, more in details, of the parallel session devoted to chemistry. We also offer some provocative reflections on the experience of on-line teaching. -
Il bilanciamento delle reazioni di ossidoriduzione: L’approccio termodinamico
The balancing of redox reactions represents one of the main difficulties encountered by students in the teaching of basic chemistry. This problem can be addressed differently depending on the academic course and the function, complementary or basic, exerted by the chemical disciplines within the course itself. This article focuses on the use of the thermodynamic method as a versatile and flexible teaching tool for balancing redox reactions in academic studies. Strengths, possible criticalities, and application methods of this approach will be discussed using redox reactions in aqueous solution.
Percorsi storico epistemologici per la scuola superiore
-
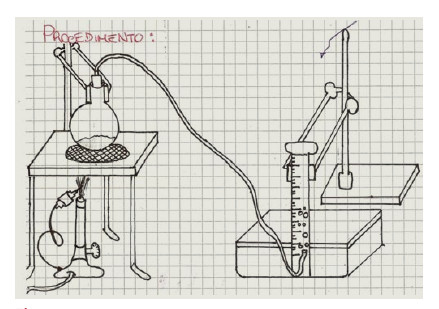
La nascita del concetto di gas: Un percorso didattico
This study programme was developed at the A. M. Enriques Agnoletti Scientific High School and it is the result of the collaboration of four natural science teachers. It was directed to students specializing in mathematics and applied sciences of the second year classes (age range 15-16 years).
Teamwork at the planning stage, compresence during class activities and a shared analysis of results obtained are characterising elements of the action research method which our department of natural science has been committed to for several years.
The concept of gas was introduced by making observations about the materiality of air. In fact, prior knowledge of the latter, in this age group, should not be taken for granted. On the basis of these observations, a study path was initiated through narration and simple experiments, enabling pupils to recreate the history of the process which led early
chemists to the discovery of fixed air. This approach to the study of chemistry is profoundly different from the classic method used in textbooks insofar as its objective is to encourage pupil - teacher interaction in the realisation of concepts and does not limit itself to providing ready-made definitions.
Percorsi laboratoriali
-
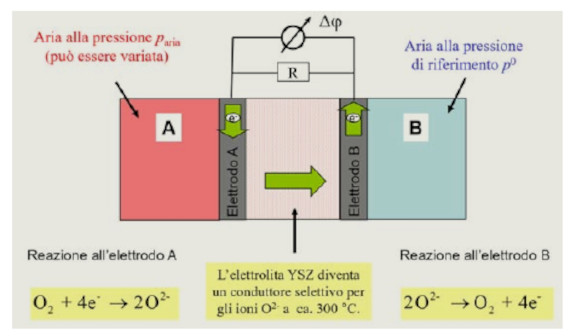
Misura diretta del potenziale chimico del diossigeno con una sonda lambda
We present an experiment for the determination of the pressure dependence of the chemical potential of dioxygen using a probe usually found in automobile combustion engines. The experiment is adapted to the needs of the high school in terms of cost and the ancillary equipment required; the apparatus is designed to promote clarity and educational understanding.
Measurements show the logarithmic connection between the pressure of the gas and its chemical potential. The data are then successfully compared quantitatively with the theoretical prediction. -
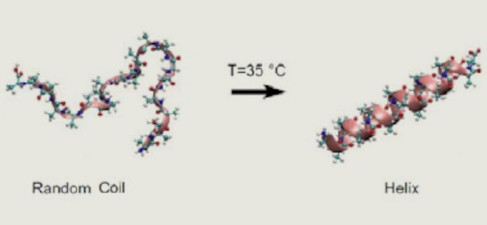
Proprietà viscosimetriche della gelatina di collagene
Le misure al viscosimetro rotazionale con accurato controllo della temperatura sono capaci di caratterizzare il comportamento reologico della gelatina di collagene. L’organizzazione dell’apparato sperimentale e le procedure di misura adottate permettono lo studio didattico (in particolar modo nella scuola secondaria ad indirizzo chimico) di un materiale polimerico d’uso comune la cui manipolazione è completamente innocua, mettendo in luce una ricca galleria di comportamenti di chimica-fisica dei polimeri.